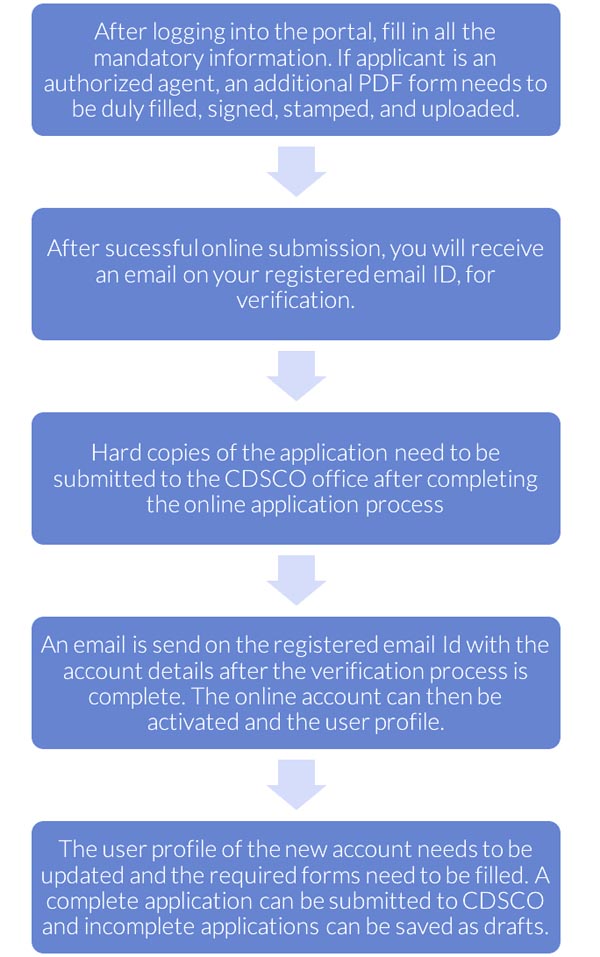
About CDSCO “SUGAM” online portal
All regulatory submissions to CDSCO whether a Fresh application, Endorsement Application, Renewal , Change notifications etc are expected to be submitted through an online account on the SUGAM portal of CDSCO. The Sugam account can only be created in the name of the applicant i.e. the Indian Entity in whose name the application is submitted to CDSCO.
One applicant can have only one account on SUGAM portal of CDSCO. It is important to prepare this account with correct and complete credentials of the applicant.
The process can take anywhere between 5 to 10 working days and includes submission of certain documents to CDSCO verification team for final activation of the account.
CosmeticsConsultants.in team works closely with CDSCO respective divisions and CDSCO IT cell to activate the SUGAM account in favor of the applicant.
How to apply on CDSCO “SUGAM” online portal
To have access to the Online Registration Portal for CDSCO, the following steps should be followed:
- Using any of the standard web browsers such as Firefox, Google Chrome etc., go to the link http://cdscoonline.gov.in
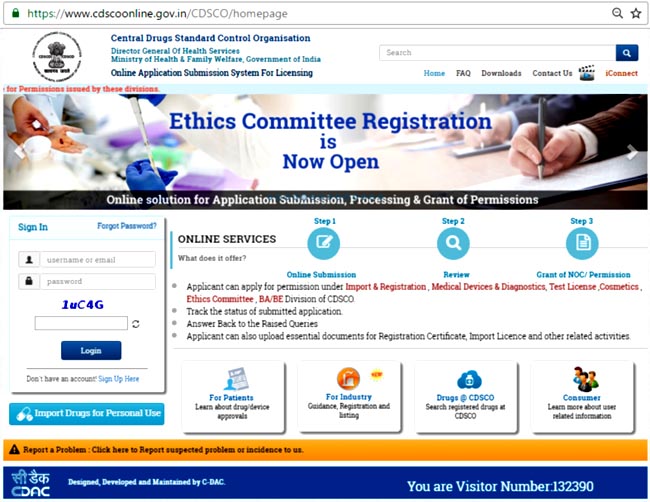
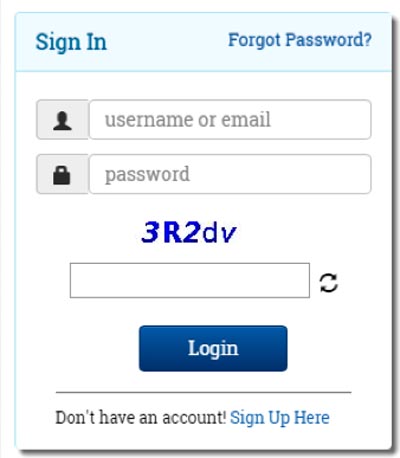
- Click on “Sign up here” to get registered (Figure 1). If you already have an account, you can Login using the Id and Password. (Figure 2).
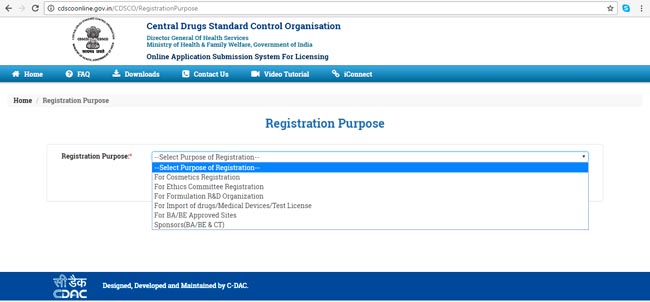
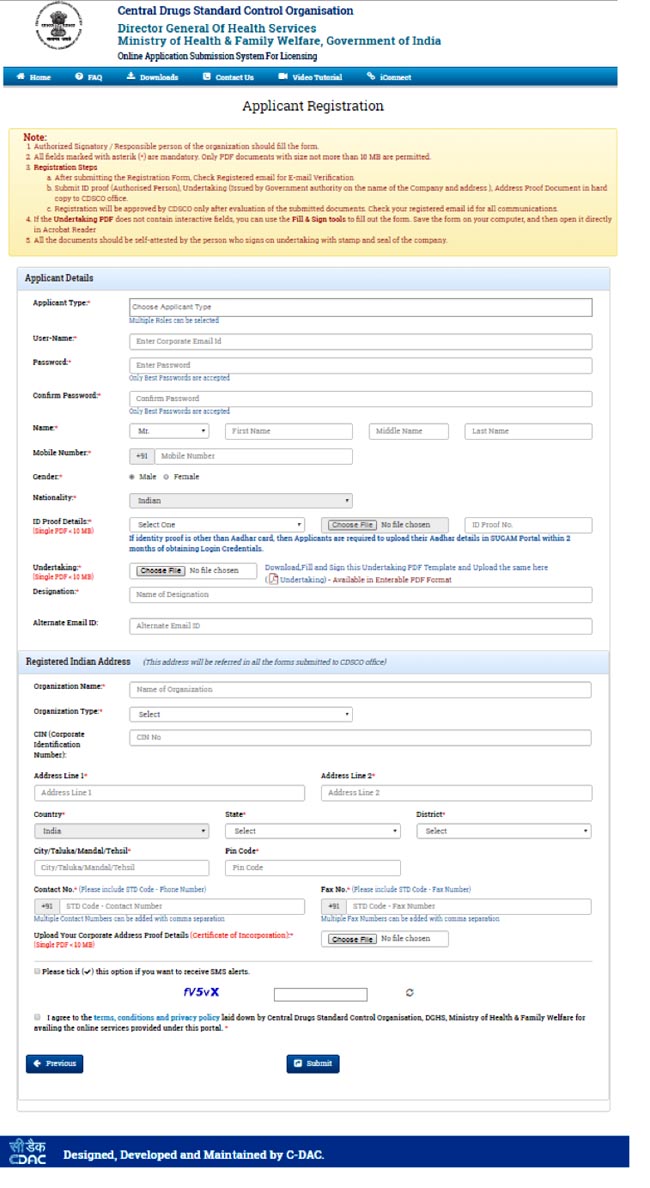
- Select the registration purpose (Figure 3) and fill the Applicants details & registered Indian Address (Figure 4)
- Login through the user account
- Open the list of all the licenses that can be controlled
- By just a few clicks, options such as licenses issued, suspended and cancelled; manufacturing facility for each location, details of import licenses, technical staff working at each facility etc. will appear right on the screen.
Documents required for CDSCO “SUGAM” Registration
- ID Proof (Aadhar card, PAN Card, Passport)
- Undertaking in given format.
- Address proof details. (Copy of CIN, IEC)
This initiative by the CDSCO has found immense acknowledgement and is a praiseworthy step in the direction of client empowerment. The portal SUGAM will make the process faster, easier and nearly paperless for everyone involved- importers, manufacturers, agents, companies and the CDSCO itself.
Cosmetics Import Registration Application Dossier

Step 1: Gathering Primary information.
Before we set out to prepare the regulatory documents, we need to identify the “ ENTITY & SKU Framework” for the regulatory submission. It is important to critically examine the following “Primary” questions.
1. Name and address of the Brand holder for all products.
CosmeticsConsultants.in - Professional Tips: Cosmetic registration in India is ENTITY DRIVEN. This is important to investigate correctly as the application for registration of cosmetics will be made in the name of this ENTITY. A mistake here due to incorrect or incomplete name and/or address can mean RE-DOING the entire regulatory submission and paying the fees again.
2. In case, the Exporter of products to India is a separate entity/different from Brand Holder, Name and address of the Exporting Company
CosmeticsConsultants.in - Professional Tips: It is important to ascertain if the final export to the Indian Importing entity will be done by the Brand Hodler company or some other company. A confusion here can lead to supply chain challenges and roadblocks in custom clearances in India even after a successful grant of registration certificate for imported cosmetics.
3. A copy of the Manufacturing License or GMP Certificate or ISO certificate or Hygiene License in English version of all actual Manufacturing sites of the products
CosmeticsConsultants.in - Professional Tips: Cosmetic registration in India is ENTITY DRIVEN. It is important to pull out the names and addresses of the ACTUAL manufacturers of the products being imported in India from the manufacturing licenses issued to such manufacturers by the regulatory authorities in their respective countries. Missing out on a manufacturing site or using an incorrect or incomplete detail of an actual manufacturing site in the application for registration is considered a MAJOR regulatory change.
4. The final list of products and pack sizes intended to be registered in below format in an excel sheet
a) The Product list must include all sample sizes, testers, variants, shades, Flavors, and colors that are planned to be exported in India.
b) Please also include any different presentation of the products such as Kits, Combos, Travel set, Trial set, and Collection sets.
c) Kindly note each different presentation or size of the product must be registered in order to Import hence all the said sizes must be included in the above sheet for Registration.
CosmeticsConsultants.in - Professional Tips: Cosmetic registration in India is PRODUCT Driven. It is important to prepare an exhaustive list of products intended to be imported in India. Making a mistake or missing out on an SKU can be considered as MINOR or MAJOR regulatory change with corresponding regulatory actions required.
Case of Missing product: Considered a new product, Major regulatory change
Case of Incorrect or incomplete Name: Considered a new product, Major regulatory change
Case of New shade in the registered product: Considered a new product, Major regulatory change
Case of New Sizes in a registered product: Minor Regulatory change
Therefore as you can see, It is important to set in motion a practice of regulatory forecasting so that regulatory submissions made to CDSCO for seeking approval can be for an updated list of SKUS as desired by the business owners.
5. Artworks of all products as per the above list in PDF Files.
CosmeticsConsultants.in action: Once our team receives the artworks from the client, we go through the label claims and intended uses of each product/SKU in order to correctly categorize the product as per the categories made available by CDSCO for registration. Correct categorization as per product families will help us estimate the applicable government registration fee which needs to be deposited to CDSCO before submission of application.
Once CosmeticsConsultants.in team has answers to the above 5 questions, we set in motion an “ ENTITY & SKU Framework” that basically freezes the boundaries of THAT respective regulatory dossier. This is important to be done immediately as mistakes here can lead to minor-major or complete regulatory re-submission which can be financially expensive and time spending exercise.

Step 2: Preparing Legal Documents(Entity Specific)
Once Our team has received the information in Step no 1, we will start working on the first draft versions or steps pertaining to the Legal documents that are required to be prepared.
1. Power Of Attorney
An overseas Brand holder/ Manufacturer has to issue a Power of Attorney to an Indian Entity as per the format prescribed under the Drugs & Cosmetics Act 1940. Team CosmeticsConsultants.in will use the information received in Step no 1 to prepare the first draft version of the Power of Attorney format and share it with the client for its approval.
CosmeticsConsultants.in - Professional Tips:
– Power of attorney has to be issued by Legal Manufacturer on its letter head signed and stamped
– Should list an exhaustive product list as per categorization explained above
– Complete correlation in product names – descriptions – pack sizes in ml or gms as per Artworks of products
– Should be Co-Jointly signed by Both Indian importer and Overseas Legal Manufacturer
– Validity of Power of attorney should be mentioned as 3 years minimum, since registration is valid for 3 Years
Common Mistake that can be avoided:
– The name of the Brand is not mentioned in the product list.
– Attestation done by the overseas Brand holder is not as per CDSCO guidelines as below.
The Power of attorney can be attested in ANY ONE of the below 3 ways:
– In the country of origin – Apostille
– In the country of origin- Indian Embassy Attestation
– In India – Attested by First class magistrate in India
2. Certificate of Free Sale
An overseas Brand holder/ Manufacturer has to procure a Certificate of Free Sale for the products that are expected to be imported into India.Since Certificates of Free Sale are issued by different bodies in different countries of the world therefore there is NO set format for the same. Accordingly, to ensure accuracy, Team CosmeticsConsultants.in shares a final product list framework that needs to be used to apply for the Free Sale Certificate.
Team CosmeticsConsultants.in will use the information received in Step no 1 to prepare the first product list version that has to be used to procure a Certificate of Free Sale by the overseas Brand Holder or Manufacturer, duly Apostilled/attested by Indian Embassy issued by either the Health Ministry of the said country or Chamber of commerce.
CosmeticsConsultants.in - Professional Tips:
– FSC can be procured EITHER by the Legal Manufacturer OR the Actual manufacture in their respective countries.
– FSC can be issued by either the Chamber of Commerce or Ministry of Health.
– FSC can be duly apostilled or attested by the Indian embassy.
– Product names on POA/FSC Must be identical and request you to use enclosed FSC List for the issuance of FSC.
– FSC must mention that products are FREELY SOLD In the country of Origin
Common Mistake that can be avoided:
– FSC uses the word “can be exported” and not “Freely sold”
-Brand name of the products are missing from the product list section
– In case of FSC issued in a language other than English, there is no English translation either attached or attested.
3. Manufacturing License
An overseas manufacturer has to produce a copy of the Manufacturing License or GMP Certificate or ISO certificate or Hygiene License issued to its manufacturing premises by the regulatory authority of its country respectively.
Team CosmeticsConsultants.in will use the information received in Step no 1 and check the validity of these documents shared by clients and suggest the next steps.
CosmeticsConsultants.in insights:
– The document needs to be either issued by the Regulatory Authority of manufacturer or
– In the case of an actual manufacturer, where no such license is granted by a regulatory authority in its country, a self-declaration conforming to GMP will be sufficient.
– Can be either notarized or apostilled or attested by Indian Embassy
– Make sure the GMP is issued or translated to the English Language.- Once the document is issued then the same needs to be attested.
Common Mistake that can be avoided:
– The license being used is either expired before submission of the application or getting expired after submission but before the grant of approval of registration certificate.

Step 3: Preparing Technical Documents (Product Specific)
1. Ingredient List
The cosmetic division of CDSCO expects a Qualitative & Quantitative breakdown of the Ingredient List of all products to be submitted in order to check the safety of products before granting product approval.
Cosmetic Products have to comply with the specifications prescribed under Schedule S and Schedule Q or any other standard of quality and safety of CDSCO & BIS from time to time. No cosmetic shall be imported unless it complies with the specifications prescribed under Schedule S and Schedule Q or any other standards of quality and safety applicable to it, and other provisions under the rules.
Schedule Q : Contains a list of permitted dyes and pigments in soap and cosmetics as below:
IS 4707 (Part 1) Ingredients Generally recognized as Safe (GRAS) given in dyes, colors and pigments
IS 4707 (Part 2) Ingredients Generally not recognized as safe (GNRAS)
a) Annex A – List of substance which must not form part of the composition of cosmetic products.
b) Annex B – List of substances which cosmetic products must not contain except subject to the restrictions and conditions laid down.
c) Annex C – List of preservatives which cosmetic products may contain.
d) Annex D – List of U.V. filters which cosmetic sunscreen products may contain.
CosmeticsConsultants.in insights:
– Needs to be Qualitative & Quantitative Ingredient List with the exact composition of ingredients totaling up to 100%
– CDSCO will check the maximum and Minimum Permissible Limits of each ingredient as per IS 4707:2017 of Bureau of Indian standards
– Chemical Abstracts Services No (CAS No.) should be mentioned so that CDSCO can refer to the same.
– Functions of ingredients should be listed for each ingredient.
– List of Ingredients mentioned on the Composition Sheet Must correlate with List of Ingredients on Artworks
Common Mistake that can be avoided:
– The product name on this document must be exactly the same as per POA and Free sale certificate
– The document must be issued on Letterhead of Legal or Actual Manufacturer duly signed and stamped stating the name and designation of the person who has signed the document.
2. Certificate of Analysis
The Certificate of Analysis of all products have to be submitted to the Cosmetic division of CDSCO as part of regulatory dossier accompanying application for registration of cosmetics.
Cosmetic Products have to comply with the specifications prescribed under Schedule S and Schedule Q or any other standard of quality and safety of CDSCO & BIS from time to time . No cosmetic shall be imported unless it complies with the specifications prescribed under Schedule S and Schedule Q or any other standards of quality and safety applicable to it, and other provisions under the rules.
Schedule S: Finished products specific standards as per Bureau of Indian Standards. The following cosmetics in finished form shall conform to the Indian Standards specifications laid down from time to time by BIS (+30) Skin Powders. / Skin Powder for infants./ Tooth Powder./ Toothpaste./ Skin Creams. / Hair Oils. /Shampoo, Soap-based. / Shampoo, Synthetic-Detergent based. / Hair Creams. / Oxidation hair dyes, Liquid. / Cologne./ Nail Polish (Nail Enamel). / After Shave Lotion. / Pomades and Brilliantines. / Depilatories Chemical. / Shaving Creams. / Cosmetic Pencils. / Lipstick. / Toilet Soap. / Liquid Toilet Soap./ Baby Toilet Soap. / Shaving Soap. / Transparent Toilet Soap./ Lipsalve / Powder Hair Dye / Bindi (Liquid). / Kum Kum Powder / Henna Powder …etc.
CosmeticsConsultants.in insights:
– Product name on Certificate of analysis must be identical to name on Power of attorney and Free sale certificate.
– Certificate of analysis must mention the columns such as Specification, Test methods and Results for each Parameter which is Being tested.
– Bureau of Indian Standards has issued Relevant Indian Standards that need to be followed.
– The mentioned methods for each category is listed by CDSCO and manufacturer must use the applicable test in the analysis document to showcase the results
– The document must be issued on Letter head of Legal or Actual Manufacturer duly signed and stamped stating the name and designation of the person who has signed the document.
3. Test Methods & Specifications
A Brief write up of Test Methods & descriptions of Specifications used to perform the tests listed in Certificate of Analysis needs to be submitted to CDSCO. Common tests are such as pH, Viscosity Heavy Metal tests or any other internal methods. These should be duly signed & stamped by the authorized personnel of the manufacturer.
4. Heavy Metals in Cosmetics
The Bureau of Indian Standards has laid our clear verdict on how much heavy metals are allowed in India.
All cosmetic products being placed in the Indian Markets need to follow the below specifications for heavy metals.
| Test Items | BIS Limits | Test Methods |
| Arsenic (As) | < 2 ppm | ICP/MS* |
| Lead (Pb) | < 20 ppm | ICP/MS* |
| Mercury (HG) Eye-Area Products Such As Eyeliners And Mascara | < 70 ppm | ICP/MS* |
| Other Finished Cosmetic Products | < 1 ppm | ICP/MS* |
| Other Heavy Metals (Cd, Cr, Ni) | < 100 ppm | ICP/MS* |
*Inductively Coupled Plasma Mass Spectroscopy
CosmeticsConsultants.in - Professional Tips:
– A Declaration stating that the products are free from Hexachlorophene content needs to be submitted.
– The overseas manufacturer can also submit a declaration confirming that the products being exported to India follow the above guidelines of BIS with regards to Heavy metals.
– No cosmetics shall be imported in India on which lead or arsenic compound has been used for the purpose of coloring.
– The above reports or undertaking needs to be signed and stamped by the authorized personnel of the manufacturer.
5. Non Animal Test Declaration
Animal testing on cosmetics is Banned in India. The manufacturer should submit s self declaration undertaking that the products being imported in India have NOT been tested on animals on or after 12.11.2014.
This undertaking needs to be signed and stamped by the authorised personnel of the manufacturer.
6. Declaration on Non Human Ingredient/cells/tissues usage
Ingredients derived from Human cells or tissues are banned from use in Cosmetics in India. Therefore, CDSCO expects manufacturers to declare and undertake that
“none of the ingredients are derived from any human cells or tissues and all ingredients are safe to be used in cosmetics”
This undertaking needs to be signed and stamped by the authorised personnel of the manufacturer.
7. Declaration for Use of Microbeads
CDSCO India has banned the use of non-biodegradable Microbeads in cosmetic products the size of which is less than or equal 5mm. Therefore a declaration from the manufacturer to this effect needs to be submitted.
This undertaking needs to be signed and stamped by the authorised personnel of the manufacturer.
8. Brief write up of the Brand, List of Partners/Directors & Export countries
CDSCO expects us to submit a brief write up on information of Brand covering information on topics such as Company History Brief about company activities and products, Directors Names and address , Export activities and authorisations, or if any Certifications or awards received etc.
9. Petrolatum
Various subjective declarations such as Information regarding refining history of petrolatum if petrolatums is used as an ingredient in any of the product.
10. Sodium Hyluronate/ Hyluronic Acid
In case product contains Ingredients such as Sodium Hyluronate/ Hyluronic Acid , then a declaration is required stating that the products do not contain any ingredient from human cells or tissues and a state- ment declaring sources of the ingredient is required
11. Labels of all products
The labels or regulatory artworks of the products are required to be submitted to CDSCO as part of documents for processing of application for grant of approval.
Under the Drugs & Cosmetics Act, strict and clear guidelines are laid out for overseas brands and importers to follow on the product packaging.
12. Checklist of Labels:
For a detailed understanding of labeling requirements as per Drugs & Cosmetics Act, please read this link.
CosmeticsConsultants.in - Professional Tips:
In Addition to the above, Cosmetic products are Packaged Commodities, therefore they are also regulated under the Legal Metrology (PACKAGED COMMODITIES) Rules 2011. Various aspects of India specific labelling & mandatory declarations need to be followed on the product labels before the products are placed on the Indian Markets. Cosmetic Products have to follow the mandatory declarations on label requirements as per both the Drugs & Cosmetics Act as well as the Legal Metrology (PACKAGED COMMODITIES) Rules 2011.
COSMETICS CONSULTANTS INDIA PTY – KNOWLEDGEABLE & RESOURCEFUL
Preparing regulatory documents is a time consuming and meticulous exercise. As an Importer or Brand Owner, you must be already engrossed in various business aspects prior to launch of a brand in India.
An incorrect understanding of how to apply for the Cosmetics Import Registration or if a mistake is made, it can lead to complete delay in business plan, overheads, financial planning issues as well as supply chain disruption.
We provide the foreign manufacturers or their Indian Agents our timely services of perfect Cosmetics Regulatory Compliances, Imports Registrations and Marketing Consultancy with unquestionable professional ethics and up-to-the-minute knowledge and resources.