PACKAGING AND LABELLING OF COSMETICS
Packaging is an activity which includes designing and producing the container or wrapper of the product. Labeling involves providing verbal information about the product and the seller. Nowadays Packaging and Labeling has become very significant as it is being used as a marketing tool to attract the consumers. There are possibilities that sellers try to deceive and mislead the consumers through packaging and labeling. In order to prevent the exploitation of consumers and to standardize the bare minimum material information the government has enacted the Consumer Protection act, 1986. The Act outlines the six major rights of the Consumers. Out of which, ‘Right to Information’ and ‘Right to Consumer Education’ are the major rights which the sellers or manufacturers are supposed to keep in mind while Packaging and labeling their products. Packaging is also done from the point of view of creating a trademark for the products.
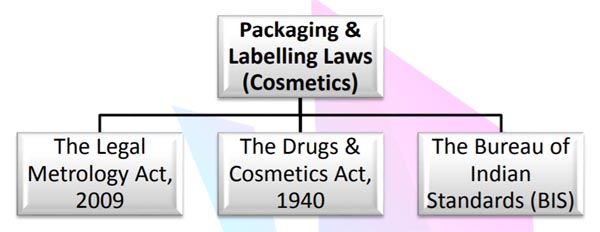
LAWS APPLICABLE
Packaging and Labeling Laws applicable specifically in case of ‘Cosmetics’ are as follows:
The Legal Metrology Rules, 2011 which was passed under section 52(2) of The Legal Metrology Act, 2009 provides for the general packaging rules for the packaged commodities. Since the key focus area of the study is ‘Cosmetics’, hence the relevant applicable laws are The Drugs & Cosmetic Rules passed under section 12 and 33 of the Drugs and Cosmetic Act and supplementary labeling guidelines requirement as provided under relevant BIS2.The BIS is national standards authority of India which formulate, recognize or promote the Indian Standards.
GENERAL REQUIREMETS UNDER THE LEGAL METROLOGY (PACKAGED COMMODITIES) RULES, 2011 AND DRUGS & COSMETICS RULE
The following table represents the general categories of labeling contents, manner of its declarations and the pictorial examples thereto:
| Categories | Manner of declarations | Examples |
| Common or generic name of the product. | • Name of Cosmetics on both inner and outer labels • if package has more than 1 product, name (and number of quantity) of each product to be mentioned on package. |
Yardley London Body spray for Men |
| Name & address of Manufacturer | • On both inner and outer labels • “Manufactured by….” Or “Packed by…” • Name of the Manufacturer. • Complete address of the premises where the product was manufactured • Additionally, name and address of the packer or/and importer in relevant cases. • If the size of the container is small- name + principal place of manufacture+ the pin code. |
Cinthol Deo Spray -Manufacturing and marketing address. |
| Manufacture Date | • Date of Manufacture, or Prepacked or Import of commodity • Rubber stamp can be used, but without overwriting. • On both inner and outer label. |
Fogg Body Spray |
| Expiry date | • “Use before … (Month and year)”- has to be labelled or the Expiry date.3 • On both inner and outer label. |
Ayur Cleansing Milk |
| Net Quantity | • Standard unit of weight or measure • On outer Label. • Weight of wrappers/containersexcluded. • “Net Quantity”- if the commodity is not likely to vary on account of environmental conditions. • If likely to vary- “When Packed” • If package capacity is less than 10 cubic cm or less – quantity declaration to be made on tag, card, tape etc. • No Declaration required:- If the net content of the package of perfume, toilet water or the like, is less than 60 ml or 30 gm. • Area surrounding the quantity declaration should be free from printed information. (Rule 8(1) of LM (packaging rules, 2011) • The maximum permissible error, in excess or in deficiency should be as per Schedule one of L.M. (Packaging) Rules 2011. • In case of wholesale packages, the total number/ net quantity of retail package contained. |
Axe Deodorant Body Spray Pears Bathing Bar |
| Retail Sale Price | • Packages’ containing alcoholic beverages or spirituous liquor – State Excise laws applies. • For reducing MRP, a sticker with revised MRP may be affixed inclusive of all taxes. • But such sticker should not cover the MRP declaration by manufacturer or packer on the package. |
Fiama Di Wills Gel Bar Axe Deodorant Body Spray. |
| Batch number | • Batch number not required- If cosmetic is of 10 grams or 25 milliliters or less. • Soaps- instead of batch number, the month and year of manufacture may be given. |
SantoorBody Deodorant |
| Warning or Caution if hazard exists | • On the inner label. • Directions for sale use • A statement of names and quantities of the hazardous or poisonous ingredients. |
Dettol Liquid Hand Wash |
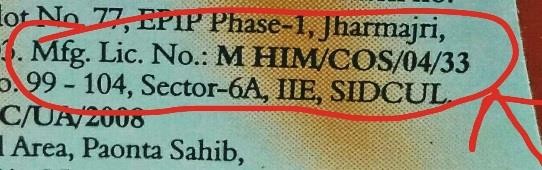
| Manufacturing License Number | Preceded by letter “M”. | Santoor Hand Wash |
| Ingredients | • Preceded by the word “INGREDIENTS” • Ingredients present in concentration of more than 1% - listed in the descending order of weight/volume. • Followed by- those in concentration of less than or equal to 1%, in any order. • Not required to appear for the packs of less than 60 ml or 30 gm. |
Ingredients of a Deodorant bottle in descending order of its volume |
| Registration Certificate Number (RCN) | • Only the label of imported cosmetics should bear RCN of the product. • The name and address of the registration certificate holder for marketing the said product in India. |
Nivea Men Deodorant |
| Consumer Care Details | • Name, address, telephone number, E-mail address • Of the person who can be contacted in case of consumer complaints. |
Santoor Hand Wash |
| Using Stickers | • Not permissible for altering or making declaration. • Except in the case of reducing MRP, but same should not cover the MRP declaration made by the manufacturer/ packer. |
(Bar code of individual packs are stickered) Lifebuoy Hand wash pouch- Offer Buy 2 @ Rs. 69/- |
| Brown/Red or green dot | • For the package of soap, shampoos, tooth pastes and other cosmetics and toiletries. • Non-Vegetarian origin- Brown dot • Vegetarian origin- Green dot. |
Roop Mantra- Neem&Tulsi Soap |
| ‘Not a standard pack size under Legal Metrology(Packa ged commodities) Rules, 2011’ | • Specific Cosmetics should be packed in the standard quantities as mentioned in the Second Schedule of the said rule. • If packed in size other than that prescribed in the schedule, then it should be prominently labeled in the said manner. • Value Based packages can be sold without complying with the Second Schedule. |
TriguniEze Eats- Rasa Vada |
DOS AND DON’TS WHILE MAKING THE DECLARATIONS
DO'S:
- Declarations should appear on Principal Display Panel
- Should be legible and prominent
- Retail sale price and net quantity –painted, printed or inscribed in contrasting color.
- Declarations to be either in Hindi or English (in addition to this, other languages also allowed)
- If declarations are in the form of handwriting or hand-script, such declarations should be clear, unambiguous and legible.
- If only one label is present, such label should contain all the information required to be shown on both inner and outer labels.
- If there is an outside container or wrapper, it should also contain all the declarations.
- A label for making the declarations can be affixed on imported packages.
- If a commodity consists of number of components for the sale of a single commodity, all the declarations are required to be made on the main package or such declarations may be given on individual packages and intimation to that effect may be given on main package.
DON’TS:
- No person/ manufacturer is allowed to alter, obliterate or deface any inscription or make any mark on the container, label or wrapper of any cosmetic unless allowed by the Licensing Authority.
- The labeling or packaging of cosmetic should not convey any false or misleading claims.
- An export package cannot be sold in India unless the manufacturer or packer has re- packed or re-labeled the commodity.
- The Legal Metrology (Package Commodity) Rules 2011 do not apply to any package containing a commodity of net content less than or equal to 10 milliliter or 10 gram.
- If a commodity consists of number of components for the sale of a single commodity, then the components cannot be sold as spare parts unless all the declarations are given on individual package.
Assistance on Cosmetics Labelling
Cosmetics Consultants India Pty provides consultation for cosmetic labelling instruction in India, as most of them are unaware of the cosmetic labelling instruction and also Form COS-1 application in India.
Apply for Form COS-2 and get Approval for Cosmetic Labelling In India
The cosmetic labelling instruction is laid down under the Drugs and Cosmetics Rules, and have the following rules-
- The inner and the outer labels should contain the Name of the product along with its manufacturing address. Principal place of manufacturing and the pin code is enough if the container is small in size as per the cosmetic labelling instruction required.
- List of ingredients used in the manufacture of the product should be clearly specified on the outer label.
- ‘Directions for use’ along with any warning or caution should be mentioned on the inner label. Names and quantities of ingredients which are hazardous in nature should also be mentioned.
- A distinctive batch number, Manufacturing Date, Best Before along with manufacturing license number (if any) must be mentioned on the label as per the cosmetic labelling instruction laid down under the Drugs and Cosmetics Rules.
- For all the imported cosmetics In January, 2001, the Indian government has made it compulsory for all pre-packaged goods to contain all the above features along with its Maximum retail sales price (MRP). The MRP includes all taxes, total transport charges, commission payable to dealers, and all charges towards advertising, delivery and packing.
- The import of pre-packaged commodities such as raw materials, bulk imports, etc., that need to undergo further processing before they are sold to end consumers are not included under this labelling requirement.
Cosmetics Consultants India Pty also provides Form COS-2 application services in India which give the guidance for the label of imported cosmetics, mentioning the registration certificate number of the product and the name and address of the registration certificate holder for marketing the said product in India.
If you need consultation for cosmetic labelling instruction, Form COS-2 application in India then contact us and we shall be glad to help you.
COSMETICS CONSULTANTS INDIA PTY – KNOWLEDGEABLE & RESOURCEFUL
Preparing regulatory documents is a time consuming and meticulous exercise. As an Importer or Brand Owner, you must be already engrossed in various business aspects prior to launch of a brand in India.
An incorrect understanding of how to apply for the Cosmetics Import Registration or if a mistake is made, it can lead to complete delay in business plan, overheads, financial planning issues as well as supply chain disruption.
We provide the foreign manufacturers or their Indian Agents our timely services of perfect Cosmetics Regulatory Compliances, Imports Registrations and Marketing Consultancy with unquestionable professional ethics and up-to-the-minute knowledge and resources.