-
see Blogs & Information
- COSMETICS IMPORT REGISTRATION :
- Definition of Cosmetics
- Registration Process Overview
- Authorized Applicants
- Indian Authorized Agent
- Categorization & Fees
- Registration Guidelines 2020
- COS-1 Application Dossier
- COS-2 Registration Certificate
- Packaging & Labelling
- Testing of Samples
- Sugam Account & Dossier
- Legal Metrology Compliance
- Check List of Application
- Liasioning for Approvals
- Endorsement to Registration
- Ammendment & Re-Registration
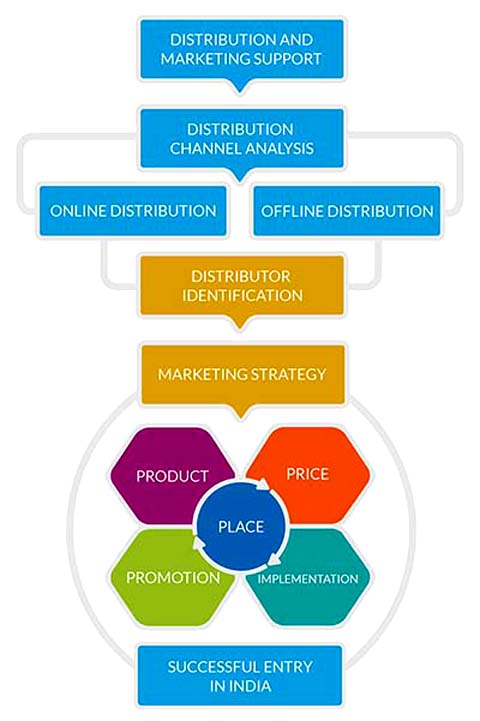
- COSMETICS MARKETING IN INDIA :
- India Cosmetics Market
- Pre-entry Market Research
- Distributors Search
- Market Entry Requirements
- Trade Shows & Exhibitions
- Corporate Entry Strategies
- Liasion/Representative Office
- India Branch Office
- India Joint Venture Office
- Wholly Owned Subsidiary